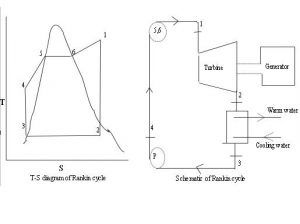
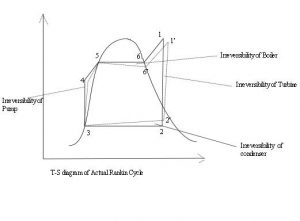
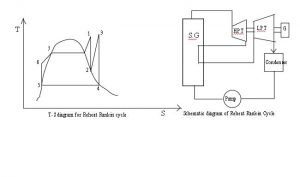
Boiler Water Treatment:
Producing quality steam on demand depends on properly managed water treatment to control steam purity, deposits and corrosion. A boiler is the sump of the boiler system. It ultimately receives all of the pre-boiler contaminants. Boiler performance, efficiency, and service life are direct products of selecting and controlling feed water used in the boiler. When feed water enters the boiler, the elevated temperatures and pressures cause the components of water to behave differently. Most of the components in the feed water are soluble. However, under heat and pressure most of the soluble components come out of solution as particulate solids, sometimes in crystallized forms and other times as amorphous particles. When solubility of a specific component in water is exceeded, scale or deposits develop. The boiler water must be sufficiently free of deposit forming solids to allow rapid and efficient heat transfer and it must not be corrosive to the boiler metal.
1 Deposit Control
Deposits in boilers may result from hardness contamination of feed water and corrosion products from the condensate and feed water system. Hardness contamination of the feed water may arise due to deficient softener system. Deposits and corrosion result in efficiency losses and may result in boiler tube failures and inability to produce steam. Deposits act as insulators and slow heat transfer. Large amounts of deposits throughout the boiler could reduce the heat transfer enough to reduce the boiler efficiency significantly. Different type of deposits affects the boiler efficiency differently. Thus it may be useful to analyse the deposits for its characteristics. The insulating effect of deposits causes the boiler metal temperature to rise and may lead to tube-failure by overheating.
2 Impurities Causing Deposits
The most important chemicals contained in water that influences the formation of deposits in the boilers are the salts of calcium and magnesium, which are known as hardness salts. Calcium and magnesium bicarbonate dissolve in water to form an alkaline solution and these salts are known as alkaline hardness. They decompose upon heating, releasing carbon dioxide and forming a soft sludge, which settles out. These are called temporary hardness-hardness that can be removed by boiling. Calcium and magnesium sulphates, chlorides and nitrates, etc. when dissolved in water are chemically neutral and are known as non-alkaline hardness. These are called permanent hardness and form hard scales on boiler surfaces, which are difficult to remove. Non-alkalinity hardness chemicals fall out the solution due to reduction in solubility as the temperature rises, by concentration due to evaporation which takes place within the boiler, or by chemical change to a less soluble compound.
3 Silica
The presence of silica in boiler water can rise to formation of hard silicate scales. It can also associate with calcium and magnesium salts, forming calcium and magnesium silicates of very low thermal conductivity. Silica can give rise to deposits on steam turbine blades, after been carried over either in droplets of water in steam, or in volatile form in steam at higher pressures. Two major types of boiler water treatment are: Internal water treatment and External water treatment.
4 Internal Water Treatment
Internal treatment is carried out by adding chemicals to boiler to prevent the formation of scale by converting the scale-forming compounds to free-flowing sludges, which can be removed by blowdown. This method is limited to boilers, where feed water is low in hardness salts, to low pressures- high TDS content in boiler water is tolerated, and when only small quantity of water is required to be treated. If these conditions are not applied, then high rates of blowdown are required to dispose off the sludge. They become uneconomical from heat and water loss consideration.
Different waters require different chemicals. Sodium carbonate, sodium aluminate, sodium phosphate, sodium sulphite and compounds of vegetable or inorganic origin are all used for this purpose. Proprietary chemicals are available to suit various water conditions. The specialist
must be consulted to determine the most suitable chemicals to use in each case. Internal treatment alone is not recommended.
5 External Water Treatment
External treatment is used to remove suspended solids, dissolved solids (particularly the calcium and magnesium ions which is a major cause of scale formation) and dissolved gases (oxygen and carbon dioxide). The external treatment processes available are: ion exchange; demineralization; reverse osmosis and de-aeration. Before any of these are used, it is necessary to remove suspended solids and colour from the raw water, because these may foul the resins used in the subsequent treatment sections.
Methods of pre-treatment include simple sedimentation in settling tanks or settling in clarifiers with aid of coagulants and flocculants. Pressure sand filters, with spray aeration to remove carbon dioxide and iron, may be used to remove metal salts from bore well water.
The first stage of treatment is to remove hardness salt and possibly non-hardness salts. Removal of only hardness salts is called softening, while total removal of salts from solution is called demineralization.
The processes are:-
Ion-exchange process (Softener Plant):
In ion-exchange process, the hardness is removed as the water passes through bed of natural zeolite or synthetic resin and without the formation of any precipitate. The simplest type is ‘base exchange’ in which calcium and magnesium ions are exchanged for sodium ions. After saturation regeneration is done with sodium chloride. The sodium salts being soluble, do not form scales in boilers. Since base exchanger only replaces the calcium and magnesium with sodium, it does not reduce the TDS content, and blowdown quantity. It also does not reduce the alkalinity. Demineralization is the complete removal of all salts. This is achieved by using a “cation” resin, which exchanges the cations in the raw water with hydrogen ions, producing hydrochloric, sulphuric and carbonic acid. Carbonic acid is removed in degassing tower in which air is blown through the acid water. Following this, the water passes through an “anion” resin which exchanges anions with the mineral acid (e.g. sulphuric acid) and forms water. Regeneration of cations and anions is necessary at intervals using, typically, mineral acid and caustic soda respectively. The complete removal of silica can be achieved by correct choice of anion resin.
Ion exchange processes can be used for almost total demineralization if required, as is the case in large electric power plant boilers
De-aeration:
In de-aeration, dissolved gases, such as oxygen and carbon dioxide, are expelled by preheating the feed water before it enters the boiler. All natural waters contain dissolved gases in solution. Certain gases, such as carbon dioxideand oxygen, greatly increase corrosion. When heated in boiler systems, carbon dioxide (CO2) and oxygen (O2) are released as gases and combine with water (H2O) to form carbonic acid, (H2CO3).
Removal of oxygen, carbon dioxide and other non-condensable gases from boiler feedwater is vital to boiler equipment longevity as well as safety of operation. Carbonic acid corrodes metal reducing the life of equipment and piping. It also dissolves iron (Fe) which when returned to the boiler precipitates and causes scaling on the boiler and tubes. This scale not only contributes to reducing the life of the equipment but also increases the amount of energy needed to achieve heat transfer. Deaeration can be done by mechanical deaeration-aeration, by chemical de-deration or by both together.
Mechanical deaeration:
Mechanical deaeration for the removal of these dissolved gases is typically utilized prior to the addition of chemical oxygen scavengers. Mechanical deaeration is based on Charles’ and Henry’s laws of physics. Simplified, these laws state that removal of oxygen and carbon dioxide can be accomplished by heating the boiler feed water, which reduces the concentration of oxygen and carbon dioxide in the atmosphere surrounding the feed water. Mechanical de-aeration can be the most economical. They operate at the boiling point of water at the pressure in the deaerator. They can be of vacuum or pressure type.
The vacuum type of de-aerator operates below atmospheric pressure, at about 82 °C, can reduce the oxygen content in water to less than 0.02 mg/litre. Vacuum pumps or steam ejectors are required to maintain the vacuum.
The pressure-type de-aerators operates by allowing steam into the feed water through a pressure control valve to maintain the desired operating pressure, and hence temperature at a minimum of 105 °C. The steam raises the water temperature causing the release of O2 and CO2 gases that are then vented from the system. This type can reduce the oxygen content to 0.005 mg/litre.
Where excess low-pressure steam is available, the operating pressure can be selected to make use of this steam and hence improve fuel economy. In boiler systems, steam is preferred for de-aeration because:
- Steam is essentially free from O2 and CO2,
- Steam is readily available
III. Steam adds the heat required to complete the reaction.
c:
While the most efficient mechanical deaerators reduce oxygen to very low levels (0.005 mg/litre), even trace amounts of oxygen may cause corrosion damage to a system. Consequently, good operating practice requires removal of that trace oxygen with a chemical oxygen scavenger such as sodium sulfite or hydrazine. Sodium sulphite reacts with oxygen to form sodium sulphate, which increases the TDS in the boiler water and hence increases the blowdown requirements and make-up water quality. Hydrazine reacts with oxygen to form nitrogen and water. It is invariably used in high pressures boilers when low boiler water solids are necessary, as it does not increase the TDS of the boiler water.
Reverse Osmosis:
Reverse osmosis uses the fact that when solutions of differing concentrations are separated by a semi-permeable membrane, water from less concentrated solution passes through the membrane to dilute the liquid of high concentration. If the solution of high concentration is pressurized, the process is reversed and the water from the solution of high concentration flows to the weaker solution. This is known as reverse osmosis. The quality of water produced depends upon the concentration of the solution on the high-pressure side and pressure differential across the membrane. This process is suitable for waters with very high TDS, such as sea water.
The semi-permeable nature of the membrane allows the water to pass much
more readily than the dissolved minerals. Since the water in the less concentrated solution seeks to dilute the more concentrated solution, the water passage through the membrane generates a noticeable head difference between the two solutions. This head difference is a measure of the concentration difference of the two solutions and is referred to as the osmotic pressure difference.
When a pressure is applied to the concentrated solution which is great that the osmotic pressure difference, the direction of water passage through the membrane is reversed and the process that we refer to as reverse osmosis is established. That is, the membrane’s ability to selectively pass water is unchanged, only the direction of the water flow is changed.
The feed water and concentrate (reject stream) ports illustrates a continuously operating RO system.
Recommended boiler and feed water quality:
The impurities found in boiler water depend on the untreated feed water quality, the treatment process used and the boiler operating procedures. As a general rule, the higher the boiler operating pressure, the greater will be the sensitivity to impurities. Recommended feed water and boiler water limits are shown in Table 4.2 and Table 4.3.
Recommended Feed Water Limits |
|||
| Factor | Upto 20 kg/cm2 | 21 – 39 kg/cm2 | 41 – 59 kg/cm2 |
| Total iron (ppm) | 0.05 | 0.02 | 0.01 |
| Total copper (ppm) | 0.01 | 0.01 | 0.01 |
| Total silica (ppm) | 1.0 | 0.3 | 0.1 |
| Oxygen (ppm) | 0.02 | 0.02 | 0.01 |
| Hydrazine residual ppm | – | – | 0.02-0.04 |
| pH at 25°C | 8.8-9.2 | 8.8-9.2 | 8.02-9.2 |
| Hardness, ppm | 1.0 | 0.5 | – |
Recommended Boiler Water Limits (Is 10392, Year 1982) |
|||
| Factor | Upto 20 kg/cm2 | 21 – 39 kg/cm2 | 40 – 59 kg/cm2 |
| TDS, ppm | 3000-3500 | 1500-2500 | 500-1500 |
| Total iron dissolved solids ppm | 500 | 200 | 150 |
| Specific electrical conductivity 1000 400 300
at 25°C (mho) |
1000 | 400 | 300 |
| Phosphate residual ppm | 20-40 | 20-40 | 15-25 |
| pH at 25°C | 10-10.5 | 10-10.5 | 9.8-10.2 |
| Silica (max) ppm | 25 | 15 | 10 |
Sponsored