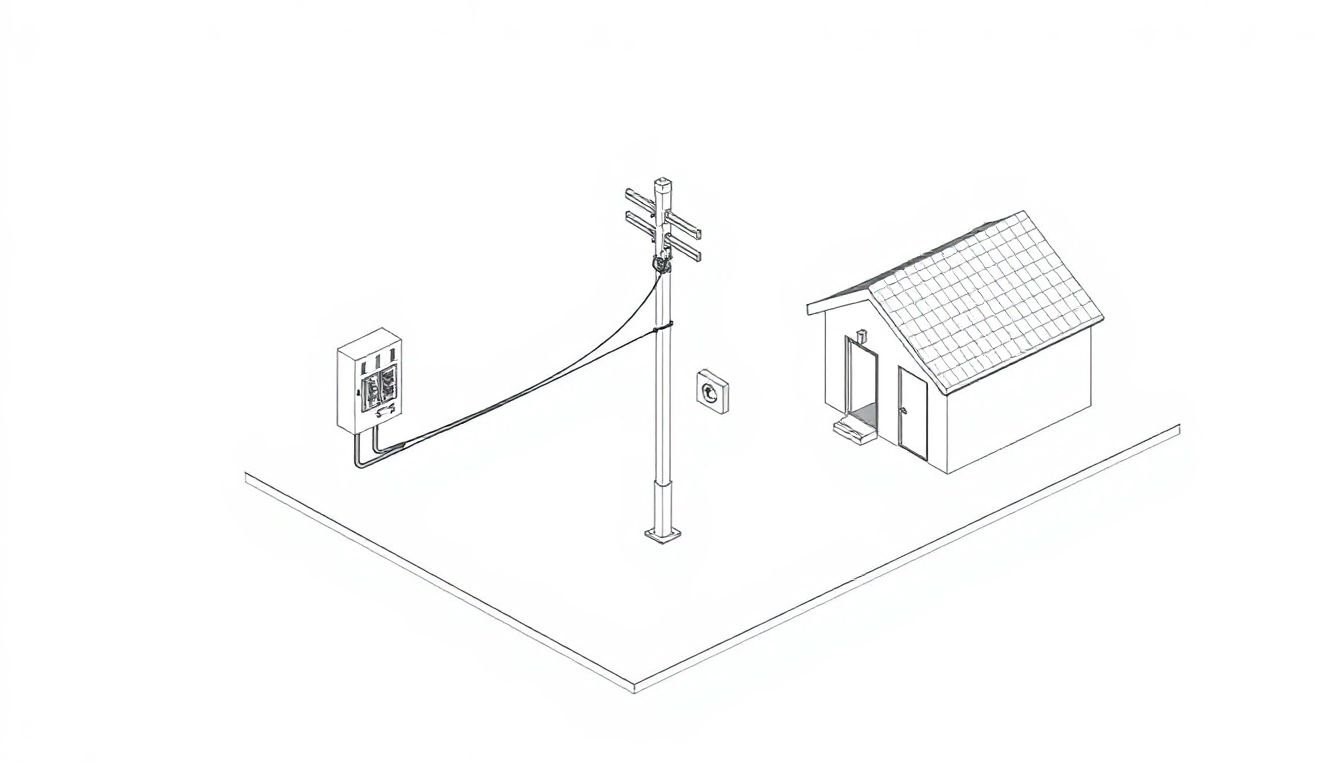
One Line Diagram Electrical
Symbolism in One Line Diagram Electrical Understanding Schematic Representation Old electrical drawings can look like a spider web. Full schematics

Symbolism in One Line Diagram Electrical Understanding Schematic Representation Old electrical drawings can look like a spider web. Full schematics
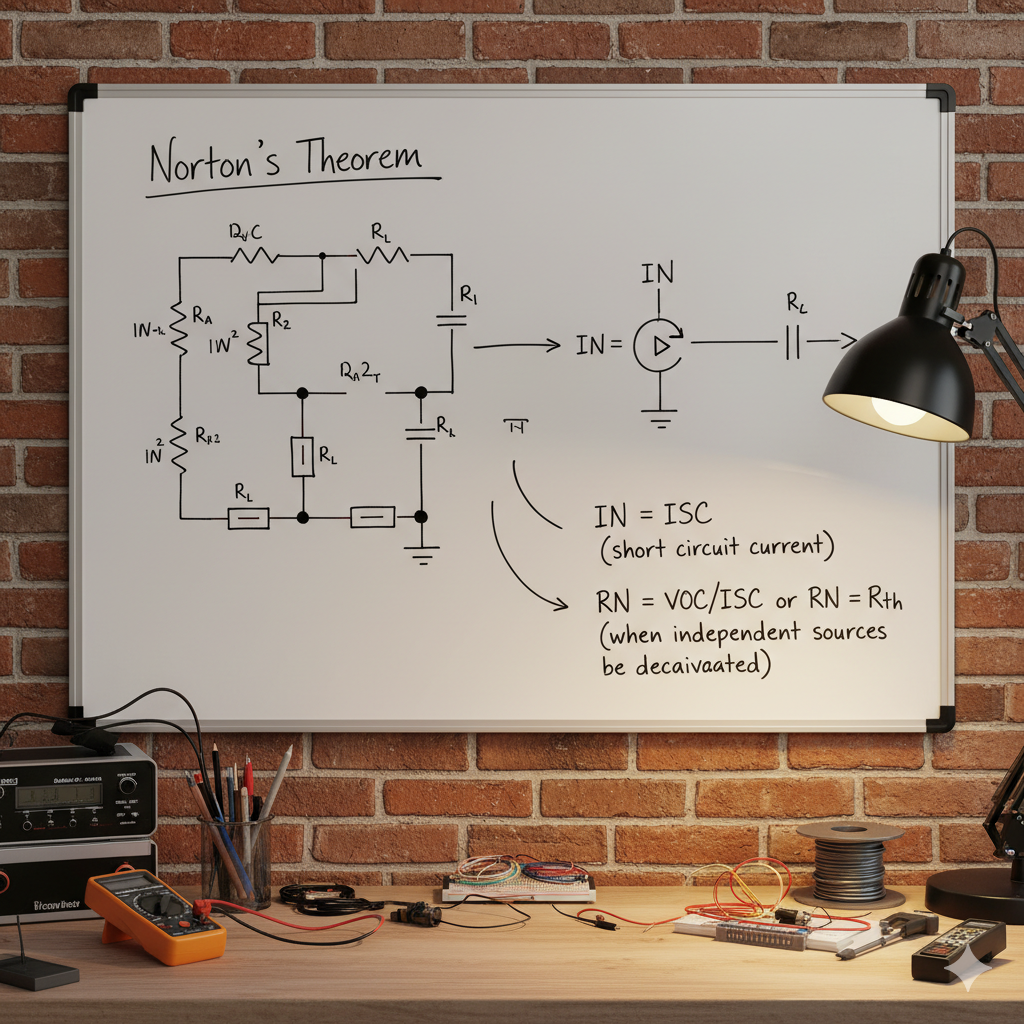
Norton’s theorem it is a fundamental concept in electrical circuit analysis that allows for the simplification of complex linear circuits.

Negative resistance is a unique and counterintuitive concept in electronics that has captivated researchers and engineers for decades. This phenomenon

Motion control forms the base for many automated tasks. It helps machines follow set paths with little error. Let’s start

Invention of the Microphone Microphone: Back in the late 1800s, folks like Emile Berliner and Thomas Edison pushed early mic
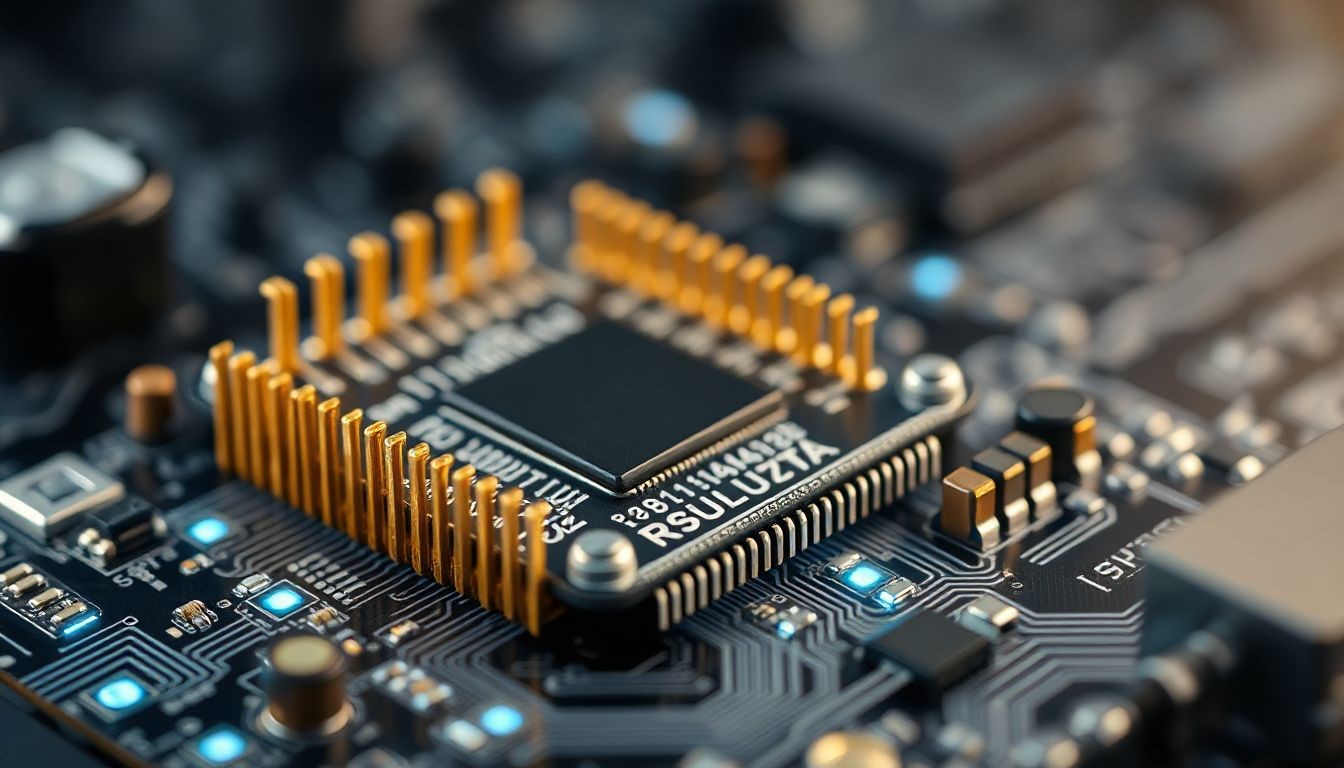
What Is a Microcontroller? A microcontroller acts like the brain in small electronic systems. It processes data and controls actions

Science is not a magic. its all About research and development for the well being of mankind.
© All Rights Reserved. MakArticles.com